Post-marketing surveillance of medicines: the Black Triangle scheme
April 2014
The European Medicines Agency (EMA) launched a new post marketing surveillance system in the Autumn called the "Black Triangle" scheme. This follows a similar scheme in the UK, where the Commission on Human Medicines (CHM) and the Medicines and Healthcare products Regulatory Agency (MHRA) encourage practitioners and patients to report any adverse effects of medicines labelled with the inverted Black Triangle logo. However, this is the first time the Black Triangle logo has been introduced onto medicines marketed in EU countries. It is hoped that this scheme will prevent the reoccurrence of previous failures of additional monitoring of high-profile drugs including Vioxx (an osteoarthritic) and Avandia (an anti-diabetic) which both remained on the market for some time before safety problems were detected.
Why additional monitoring might be needed
The clinical trial requirements for the introduction of medicines onto the current market are familiar:
- Phase 0 or I: regarding pharmacodynamics, pharmacokinetics and safety of a new or novel drug.
- Phase II: regarding the efficacy of a drug, often determined by recruiting a small cohort of healthy patients.
- Phase III: often the therapeutic confirmatory phase carried out in excess of 1,500 subjects where subject inclusion and exclusion criteria are quite strict to match the subset.
 The number of subjects recruited into a clinical trial is usually small relative to the total patient population, and the testing period (usually 6-12 months) is often short compared to the relevant course of therapy. This could potentially result in a limited picture of safety and efficacy being gathered from the clinical trials. Furthermore, once a drug is released onto the market it may be used by a patient group which includes those with additional health complications that are unaccounted for in clinical trials. These possible limitations on the data obtainable in clinical trials mean that the use of medicines over longer periods of time by a wider population can provide more comprehensive information on a drug, and its possible adverse effects can be detected from real-life experiences. Medicines that need this kind of additional monitoring, as selected by regulatory authorities, will now require the inverted Black Triangle to be displayed in their information leaflets and SmPCs.
The number of subjects recruited into a clinical trial is usually small relative to the total patient population, and the testing period (usually 6-12 months) is often short compared to the relevant course of therapy. This could potentially result in a limited picture of safety and efficacy being gathered from the clinical trials. Furthermore, once a drug is released onto the market it may be used by a patient group which includes those with additional health complications that are unaccounted for in clinical trials. These possible limitations on the data obtainable in clinical trials mean that the use of medicines over longer periods of time by a wider population can provide more comprehensive information on a drug, and its possible adverse effects can be detected from real-life experiences. Medicines that need this kind of additional monitoring, as selected by regulatory authorities, will now require the inverted Black Triangle to be displayed in their information leaflets and SmPCs.
Additional monitoring status
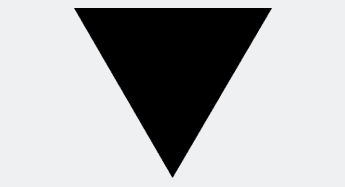
With the introduction of the EU pharmacovigilance legislation1, medicines that are subject to additional monitoring require an inverted Black Triangle logo to be displayed on package leaflets and SmPCs for selected medicines from autumn 2013, together with this wording:
▼ This medicinal product is subject to additional monitoring.
Marketing authorisation holders of affected medicines have been provided with template wording and advised to update product information to include the Black Triangle symbol with additional optional information.
Medicines that are subject to additional monitoring include:
- any medicines authorised on or after 1 January 2011 that contain a new active substance;
- medicines that are characterised as a biological medicine such as a vaccine or derived from blood;
- medicines which have been given conditional approval under exceptional circumstances; and
- medicines which require further studies i.e. need to provide data on long term use of the medicine.
Such medicines require the Black Triangle to be displayed. Furthermore, it should be noted that the MHRA or other national regulators can request already marketed medicines to be placed under additional monitoring, for later approval by the EMA's Pharmacovigilance Risk Assessment Committee (PRAC).
Medicines currently under additional monitoring in the EU were published in April 2013 by PRAC, in a list which is reviewed on a monthly basis. A medicine remains on the EMA's additional monitoring list for 5 years, or if earlier, when a PRAC review recommends that it is low risk, before it can be removed from the list.
 Advertising the Black Triangle
Advertising the Black Triangle
Since the introduction of the Black Triangle scheme, medicines which require additional monitoring are expected to provide Black Triangle status with the primary aim of encouraging practitioners and patients to report adverse effects. Specifications for the symbol are themselves key, as stated in clause 4.11 of the ABPI Code of Practice for the pharmaceutical industry:
"The symbol should always be black and its size should normally be not less than 5mm per side but with a smaller size of 3mm per side for A5 size advertisements and a larger size of 7.5mm per side for A3 size advertisements"
Additional requirements include the need for the symbol to appear once and to be located adjacent to the most prominent display of the name of the product, no written explanation being necessary. Alternatively, the following can be written "this medicine is subject to additional monitoring".
Reporting on Black Triangle medicines
In the UK, the MHRA requests that all adverse reactions experienced when using Black Triangle medicines are reported by health professionals and patients to the Yellow Card Scheme set up by the MHRA to allow authorities to analyse new emerging data and to make appropriate changes if necessary. Similar rules apply for pharmaceutical companies, who are also expected to report adverse reactions and can find further information on the requirements for managing products subject to additional monitoring by following the EMA good pharmacovigilance practices guidelines.
The introduction of the EU wide Black Triangle scheme aims to improve our understanding and reporting of current market medicines. Ultimately, pharmacovigilance is aimed to improve patient care and safety to medicines.
If you have any questions on this article or would like to propose a subject to be addressed by Synapse please contact us.

Paul England
Paul is a senior associate and professional support lawyer in the Patents group and is based in our London office.
"Medicines that need this kind of additional monitoring, as selected by regulatory authorities, will now require the inverted Black Triangle to be displayed in their information leaflets and SmPCs."

