Online sales of drugs in France
May 2015
The authorisation to sell drugs online was first implied by the ECJ in the DocMorris case (ECJ, 11 December 2003, case C-322/01) and introduced by the EU Directive 2011/62/EU of 8 June 2011, amending Directive 2001/83 on the Community code relating to pharmaceutical products for human use.
The latter was implemented in France by legislation dated 19 December 2012. However this implementation has actually been strongly resisted by local pharmacists and reveals French peculiarities. Indeed, the sale of pharmaceutical products in France is governed and restrained by two monopolies:
- the monopoly of dispensary pharmacies ("monopole officinal"), related to the retail establishment, meaning that only authorised pharmaceutical premises can sell the said products;
- the monopoly of pharmacists ("monopole pharmaceutique"), related to the person of the seller, that is to say that the products can only be sold by a licensed pharmacist.
Who can sell pharmaceutical products online? Legal obligations imposed on pharmacists to exploit a website
In France, pharmacists hold a monopoly for the retail sale of pharmaceutical products in both brick and mortar pharmacies and online. Their activity both online and within their store cannot be separated. Therefore, pure players and supermarkets are not allowed. Each pharmacy can only have one website to sell their products and a website can only be exploited by a pharmacist owner of a brick and mortar store (art. L. 5125-33 of the French Public Health Code).
The exploitation of a website aiming at selling pharmaceutical products is subject to the following:
- A pharmacy licence or an authorisation by the Minister of Health,
- The effective opening of a pharmacy (art. L. 5125-35 of the French Public Health Code), and
- The authorisation of the Regional Health Authority (Agence Régionale de Santé) (art. L. 5125-36 of the French Public Health Code).
Once the authorisation is obtained, the pharmacist must inform its professional organisation (Ordre National des Pharmaciens) on the launch of its website.
Not only must pharmacists selling pharmaceutical products online respect all the professional ethical rules (independence, professional secrecy, non-solicitation of clientele, etc.) but the content of the pharmacies' website is also regulated.
In addition to the legal obligations applicable to e-commerce, the websites exploited by pharmacists must also include (art. R. 5125-70 of the French Public Health Code):
- contact information of the national drug agency (ANSM - Agence nationale de sécurité du medicament);
- hyperlinks to the professional organisation (Ordre National des Pharmaciens) website and to the Ministry of Health website;
- the logo imposed at European level for online sales of drugs.
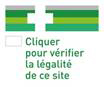

All these provisions must be complied with by all pharmacists established in other Member States and wishing to sell pharmaceutical products online in France. Of course, the pharmaceuticals products sold by pharmacists established in other Member States must ensure that said products benefit from a marketing authorization in France (art. L. 5125-40 of the French Public Health Code).
Until March 2015, pharmacists selling online had to comply with the Good Practices for selling pharmaceutical products online issued by the Ministry of Health (Ministerial Order of 20 June 2013). These Good Practices mainly concerned:
- The identification of the pharmacists and website's publisher in accordance with the law applicable to e-commerce (art. 1 of the Good Practices);
- The provision of information in relation to the price and delivery of the products and consumer rights (art. 2, 6 and 7 of the Good Practices);
- The provision of pharmaceutical advice to patients (art. 3 of the Good Practices);
- The protection of personal data transmitted by patients (art. 4 of the Good Practices).
The Good Practices also contained a prohibition against pharmacists' websites using the system of paid indexing on search engines.
On 16 March 2015, the French Supreme administrative court (Conseil d'Etat) cancelled this order considering that the Ministry had exceeded its power by imposing excessive obligations in light of the EU Directive.
Which pharmaceutical products can be sold online? Scope of the pharmaceutical products concerned by the legislation
Pursuant to EU rules, French legislation prohibits pharmacies to sell online pharmaceutical products available on prescription. Only non-prescription pharmaceutical products can be sold on pharmacies' websites (art. L. 5125-34 of the French Public Health Code).
That being said, the first draft of the French legislation attempted to limit the type of products for which online sales would be authorised. Indeed, only the online sale of pharmaceutical products so called "presented to the public", to which patients had direct access in the pharmacy, was authorised. This provision was largely limiting the scope of the products available online (only 450 products).
 Therefore, on 10 April 2013, the French Competition Authority ("Autorité de la Concurrence") rendered a negative opinion on this legislation, considering that this excessive limitation was restricting competition and was not justified by the protection of public health. The Authority thus requested the enlargement of the scope of the products which could be sold online to finally include all non-prescription pharmaceutical products and parapharmaceutical products.
Therefore, on 10 April 2013, the French Competition Authority ("Autorité de la Concurrence") rendered a negative opinion on this legislation, considering that this excessive limitation was restricting competition and was not justified by the protection of public health. The Authority thus requested the enlargement of the scope of the products which could be sold online to finally include all non-prescription pharmaceutical products and parapharmaceutical products.
Two decisions rendered on 14 February and 17 July 2013 by the French Supreme administrative court also considered that the legislation as drafted was non-compliant with European law and cancelled the provision of this legislation limiting this scope. Following these decisions, all non-prescription pharmaceutical products can now be sold online by French pharmacists.
In addition, while the sale of parapharmaceutical products on the same website as the pharmaceutical products was originally prohibited, it is today authorised for pharmacists to sell bodily hygiene products through the same website, if said products are proposed through a different tab clearly stating the nature of the products and distinguishes them from pharmaceutical products.
Illustration of the applicable regime through litigation
A decision rendered by the first instance Court of Paris (TGI Paris, 1001pharmacies.com, 8 August 2014) evidences the severity and rigidity of the French legislation.
The website 1001pharmacies.com was exploited by a company – which is not a pharmacy – selling originally only parapharmaceutical products online. Consequently, the website was not authorised by a Regional Health Agency (Agence Régionale de Santé).
However, in April 2014, the company started selling pharmaceutical products by referencing the drugs from the pharmacists which were partners of the site. The sale was in fact carried out by the pharmacists. The Court of Paris noted nevertheless the violation of the two monopolies governing the sale of pharmaceutical products in France.
Indeed, by acting as an intermediary between patients and pharmacists and proposing the sale of drugs on its website, 1001pharmacies.com violated the monopoly of the pharmacists on the sale of drugs. Since the company exploiting the site was not and could not be registered and authorised as being a pharmacist, 1001pharmacies.com also violated the monopoly of the dispensary pharmacies, since it did not own an authorised dispensary pharmacy.
Moreover, in total violation of the law, the pharmacists which were partners of the website were selling all types of drugs, including prescription pharmaceutical products (if the patient sent his/her scanned prescription). The company exploiting the website was also collecting client data relating to health and storing such data without being certified for processing such sensitive personal data.
State of legislation in 2015
 The Directive 2011/62/EU amending Directive 2001/83 also permits Member States the possibility to authorise online sales of prescription pharmaceutical products. The French legislator strongly refuses this possibility; trying to balance between the protection of public health, the historical model of pharmaceutical product commercialisation through local pharmacists benefiting from a strong monopoly, and the necessity to open this market in light of competition from abroad.
The Directive 2011/62/EU amending Directive 2001/83 also permits Member States the possibility to authorise online sales of prescription pharmaceutical products. The French legislator strongly refuses this possibility; trying to balance between the protection of public health, the historical model of pharmaceutical product commercialisation through local pharmacists benefiting from a strong monopoly, and the necessity to open this market in light of competition from abroad.
In March 2015, the French supreme administrative court cancelled the Ministerial Order of 20 June 2013 enacting the Good Practices for selling pharmaceutical products online.
The relative severity of the French legislation is evolving. It should become more flexible in order to encourage a larger number of pharmacists to sell their products online, and to enhance competition not only at the national level, but also at the European level.
If you have any questions on this article or would like to propose a subject to be addressed by Synapse please contact us.


Evelyne
Friedel
Evelyne is a partner and Head of Antitrust and Business Law (France) based in our Paris office.
"Each pharmacy can only have one website to sell their products and a website can only be exploited by a pharmacist owner of a brick and mortar store."

